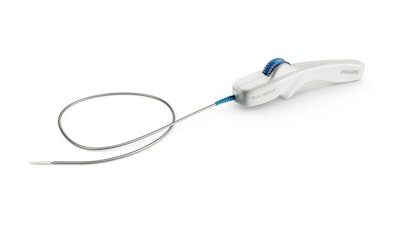
Royal Philips announced the first implant of the Duo Venous Stent System, an implantable medical device indicated to treat symptomatic venous outflow obstruction in patients with chronic venous insufficiency (CVI), following premarket approval (PMA) from the FDA.
On June 11, Dr Erin Murphy – vascular surgeon and director of the Venous and Lymphatic Program at the renowned Sanger Heart & Vascular Institute, Atrium Health, in Charlotte, N.C., and an investigator in the VIVID study, which contributed to the device’s FDA approval – successfully used the Duo Venous Stent System for the first time outside of a clinical trial.
Deep venous anatomy and obstructions can present a multitude of complexities and mechanical challenges. Engineered for the unique demands of venous anatomy and obstructions, the Duo Venous Stent System is comprised of two stents – Duo Hybrid and Duo Extend – of various sizes. Duo Hybrid has a distinct integrated design that combines multiple zones of differing mechanical properties into a single stent. For long lesions, Duo Extend smoothly overlaps with the Duo Hybrid to extend therapy. These two stents are designed to work together and minimize the risk of stent fracture and corrosion, while providing an option to stent within caudal veins with smaller diameters.
“Duo is the first stent that offers a differential design for the challenges of venous anatomy – a focal area that withstands the forces of compression as well as the flexibility to accommodate curvature of the vessel,” said Dr Kush Desai, a highly regarded Interventional radiologist and associate professor of Radiology, Surgery and Medicine at Northwestern University in Chicago, as well as a leading enroller and investigator for the VIVID study.“
 Philips
Philips






















