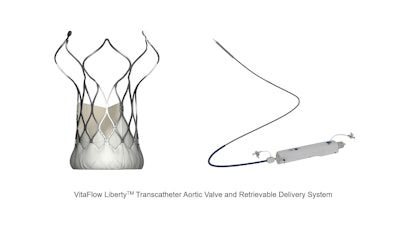
MicroPort CardioFlow recently announced that its self-developed second-generation transcatheter aortic valve implantation (TAVI) device, the VitaFlow Liberty Transcatheter Aortic Valve and Retrievable Delivery System (VitaFlow Liberty), has received EU CE-MDR certification.
The TAVI solution provided by CardioFlow helps avoid open-heart surgery and offers benefits like minimal trauma and quick recovery.
Before launching into the EU market, VitaFlow Liberty conducted pre-market clinical implantations at Galway University Hospital in Ireland, Rigshospitalet (Copenhagen University Hospital) in Denmark, and St Thomas' Hospital as well as Brighton & Sussex University Hospitals NHS Trust in the United Kingdom, and received very high appraisal from many well-known clinical professionals. Dr. Ole De Backer, a professor of interventional cardiology, who led the TAVI procedures at Rigshospitalet stated, "The overall release process of VitaFlow Liberty is notably stable, ensuring precise positioning. This stability is especially crucial in patients with a small left ventricles, where VitaFlow Liberty consistently achieves stable and precise deployment, fully demonstrating its distinct advantages. We look forward to its positive impact on a broader patient population following CE certification." It has been reported that the European post-market clinical project will also be planned to start this year.
As part of CardioFlow's global expansion roadmap, the company has also achieved milestones with CE application on three of its products, including the Alwide Plus Balloon Catheter, an essential accessory for aortic valve procedures, as well as the AnchorMan Left Atrial Appendage Closure System and the AnchorMan Left Atrial Appendage Access System, both developed by its subsidiary, CardioAdvent.






















