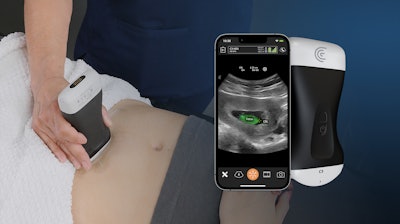
Clarius Mobile Health has obtained FDA clearance for the Clarius OB AI fetal biometric measurement tool, improving access to obstetrical (OB) prenatal monitoring and care in resource-limited areas. The OB AI model automatically performs fetal biometry measurements to estimate fetal age, weight and growth intervals and is available now with the Clarius C3 HD3 wireless handheld ultrasound scanner in the United States and Canada.
Developed with deep learning models leveraging more than 30,000 de-identified fetal ultrasound images, Clarius OB AI provides consistent and precise measurements enabling new ultrasound users, including midwives and nurses, the ability to perform accurate fetal ultrasound measurements with confidence.
Two-thirds of women worldwide lack access to prenatal ultrasound for monitoring wellbeing1. While ultrasound imaging is widely recognized as the gold standard for capturing accurate biometric measurements to monitor fetal wellbeing, the high cost of equipment, lack of portability, and the extensive training required are barriers to widespread adoption by midwives and nurses. By integrating AI with affordable and specialized ultrasound technology, Clarius aims to broaden ultrasound access for midwives and nurses, to help improve maternal and fetal health outcomes.
OB AI is included with Clarius membership. Once activated on the Clarius app while a clinician is scanning the abdomen, OB AI automatically highlights key fetal anatomy and places calipers to calculate measurements, which appear on screen and can be added to a patient's electronic record from the app. Clarius OB AI is the 8th AI model developed in-house that is available with Clarius ultrasound scanners.






















